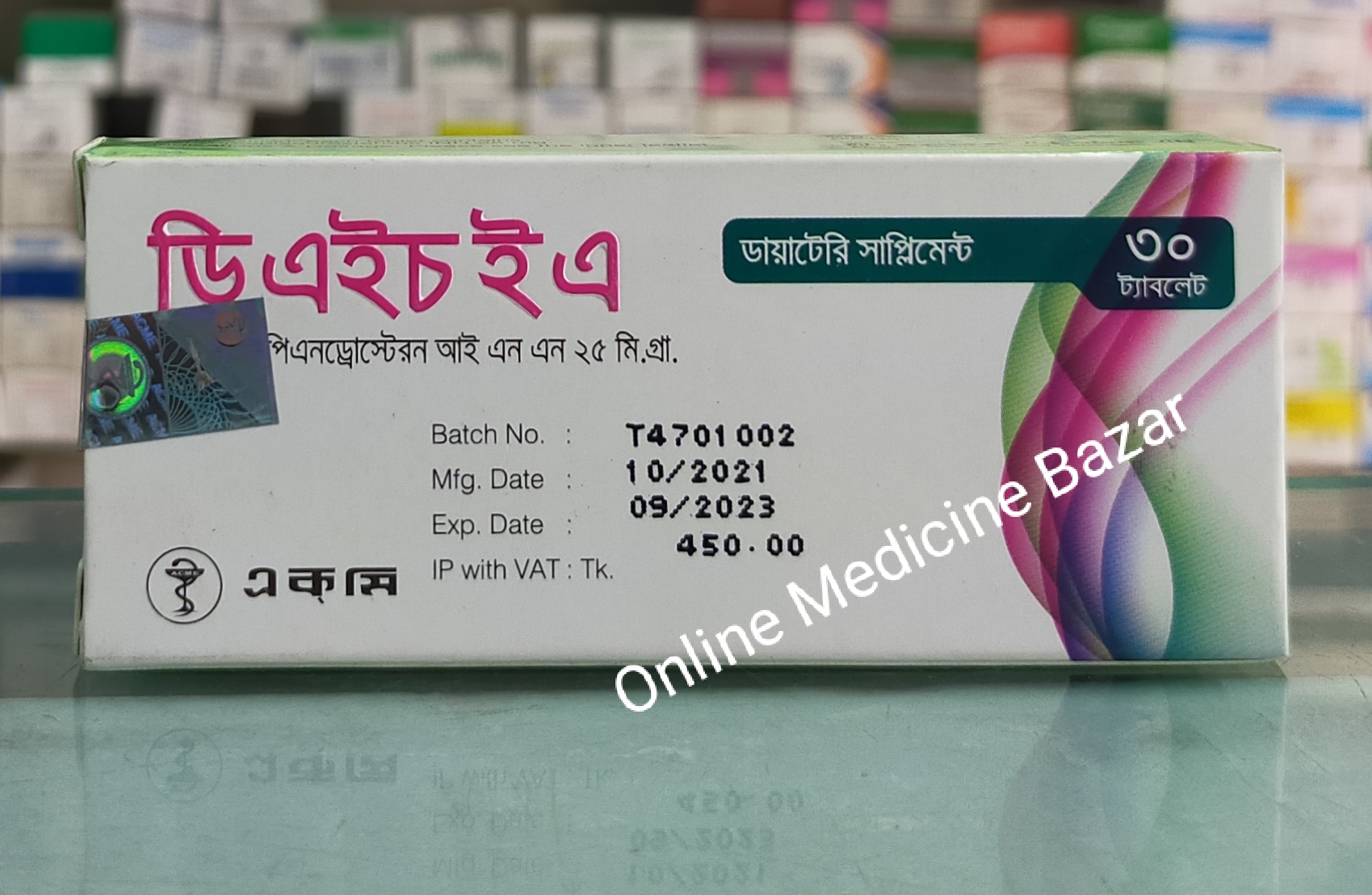
DHEA 25 mg Tablet-10's Strip
Tablet
Generics: Dehydroepiandrosterone
Brand: Acme Laboratories Ltd.৳ 150
Order By Call Or WhatsApp :
+88 01886-646186
About the Product
Full Description
Indications: Adequately powered, long-term clinical trials are lacking to support therapeutic use of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) supplementation (hereafter jointly referred to as DHEA/S). Reviews of clinical trials found no convincing evidence to support a place in therapy for DHEA in improving cognitive function or physical strength in elderly patients, or in treating postmenopausal symptoms in women, hyperlipidemia or insulin resistance, schizophrenia, or cancer. Some evidence exists to support the use of DHEA/S supplementation in women with diminished ovarian reserves, in subpopulations of elderly women with osteoporosis, and in mild systemic lupus erythematosus. DHEA is recommended as third-line monotherapy or adjunctive therapy for treatment of major depressive disorder (MDD) by Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines, and limited data suggest a potential role for DHEA as an anxiolytic.